● Intended Use
The kit intends for in vitro qualitatively detection of nucleic acid RNA of Neisseria Gonorrhoeae (NG) in genital swabs. It can be used to assist in the diagnosis of reproductive tract infectious diseases (RTIS). The detection results are only for clinical reference, and aids in the diagnosis of RTIS if used in conjunction with other clinical and epidemiological information.
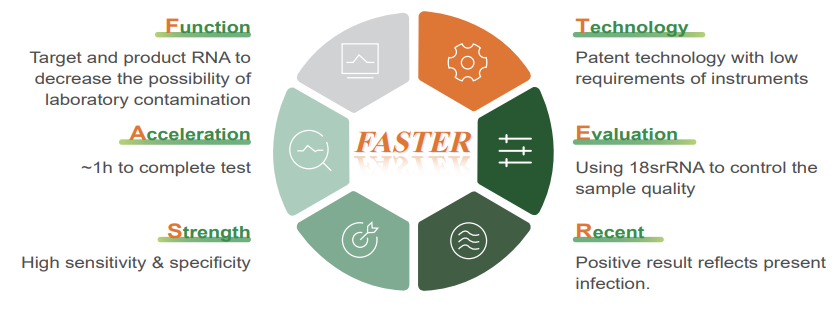
● Features
● Procedure and Equipment

● Genitourinary Tract Infections
Genitourinary Tract Infections (GUTS), which commonly caused by viruses, mycoplasma, and chlamydia, are mainly resulted by the Coinfection status of Neisseria Gonorrhoeae (NG), Chlamydia Trachomatis (CT), and Ureaplasma Urealyticum (UU). It tends to develop clinically insidiously and has a variety of complications. GUTS can lead urethritis, epididymitis, prostatitis and infertility in men; urethritis, cervicitis, pelvic inflammatory disease and infertility, ectopic pregnancy and even abortion in women.
GUTS occurs in all age groups, especially in adults and elders. WHO (2020) reported more than 20,000 elders dead due to genitourinary diseases. Globally, GUTS leads high deaths in Brazil, Mexico, and Ecuador. It is significant to detect genitourinary tract pathogens for precise treatment.
ZBI’s Neisseria Gonorrhoeae (NG) Nucleic Acid Assay adopted with the clinical requirement that improve the detection time, sensitivity, and specificity.
 ZBI-Genitourinary Tract Infection RNA Rapid Dtection.pdf
ZBI-Genitourinary Tract Infection RNA Rapid Dtection.pdf









